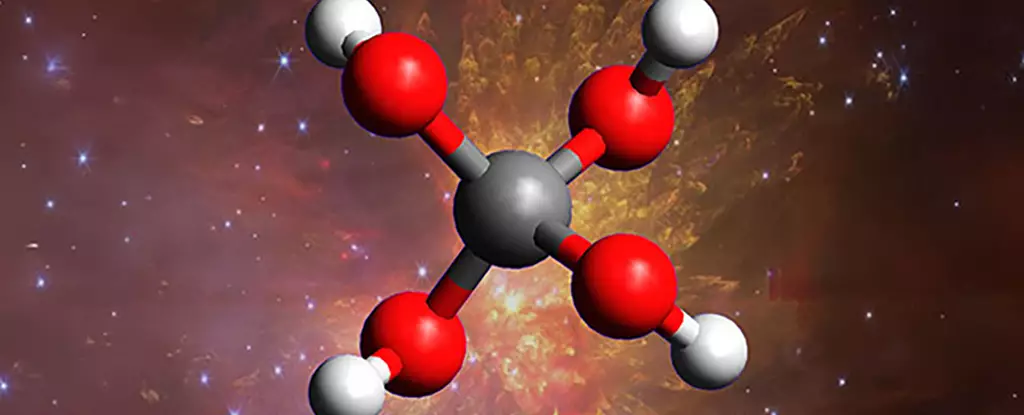
In a remarkable turn that challenges our fundamental understanding of the cosmos, scientists have successfully synthesized a molecule long theorized but never observed: methanetetrol, a “super alcohol” with four hydroxyl groups attached to a single carbon atom. This discovery is not merely a scientific milestone; it’s a revelation that compels us to rethink the intricacies of chemical processes occurring in the dark, cold depths of interstellar space. By simulating the extreme, starless environments of deep space within laboratory conditions, researchers have uncovered a hidden layer of universe chemistry that was previously dismissed as impossible or simply unknown. This achievement signals a paradigm shift—what scientists once believed to be unfeasible is now within reach, and it prompts skepticism about the limits of our knowledge.
Challenging Conventional Wisdom About Space Chemistry
The creation of methanetetrol wasn’t accidental but a calculated effort rooted in a fundamental question: What else is hiding in the shadows of the universe’s icy corners? The molecule’s instability makes it impossible to exist on Earth, yet under the conditions mimicked in laboratories—ultra-cold temperatures and high-energy radiation—it appeared for a fleeting moment, offering a tantalizing glimpse into the uncharted realms of space chemistry. This event fundamentally questions existing models that have categorized space molecules as simple or predictable. The fact that such a complex, highly reactive molecule can form under interstellar conditions suggests that the chemical landscape of space is far more dynamic and intricate than previously believed.
This discovery raises numerous questions about our current understanding. If molecules like methanetetrol can form amidst the chaos of cosmic rays and star explosions, what other bizarre, unstable compounds are lurking in the vast ocean of dust and ice? The assumption that space chemistry is limited to simple molecules like water or methane no longer holds. Instead, space appears to host an exotic milieu capable of producing molecules that defy earthly chemistry’s norms, indicating a universe teeming with surprising reactions and potential.
Implications for the Search for Extraterrestrial Life
Beyond the thrill of scientific discovery, this breakthrough bears profound implications for astrobiology and the ongoing quest to find life beyond Earth. If such complex molecules can form naturally in space, it bolsters the argument that the building blocks of life might be more common than we think. The presence of methanetetrol hints at a universe where prebiotic chemistry can flourish under conditions previously deemed inhospitable. It broadens the scope of potential biochemical pathways that could lead to life, even in the most unlikely environments.
However, skepticism is warranted. The molecule’s extreme instability raises doubts about its detection in actual interstellar space. Its rapid dissociation upon exposure to light—a process known as dissociative photoionization—means that capturing concrete evidence requires cutting-edge technology and a bit of luck. Yet, the very existence of this molecule in simulations compels a reconsideration of the potential chemical diversity in the cosmos. If our models are missing critical pathways, then our entire understanding of chemical evolution across the universe is incomplete and potentially biased toward terrestrial norms.
The Future of Space Exploration and Scientific Inquiry
This discovery underscores the importance of advancing our observational tools. With increasingly sophisticated telescopes and spectroscopy techniques, astronomers are now better equipped to hunt for signs of such elusive molecules. Detecting methanetetrol in the harsh realities of space remains a formidable challenge, but the scientific community is more motivated than ever to pursue it. Achieving this would constitute not just a validation of lab-based models but a profound leap in our comprehension of the universe’s chemical complexity.
The ongoing research into space chemistry also prompts a critical reflection on the limitations of our current scientific frameworks. Our knowledge of space molecules is perhaps the tip of the iceberg—estimates suggest less than 1% of the universe’s chemical diversity has been identified. As we push boundaries with innovative instruments and experimental techniques, we inch closer to unveiling a universe teeming with molecules that defy our earthly conventions and scientific assumptions. This paradigm shift challenges us to remain humble and open-minded, recognizing that the universe’s full chemical tapestry is far richer—and potentially more surprising—than our current theories suggest.

Leave a Reply