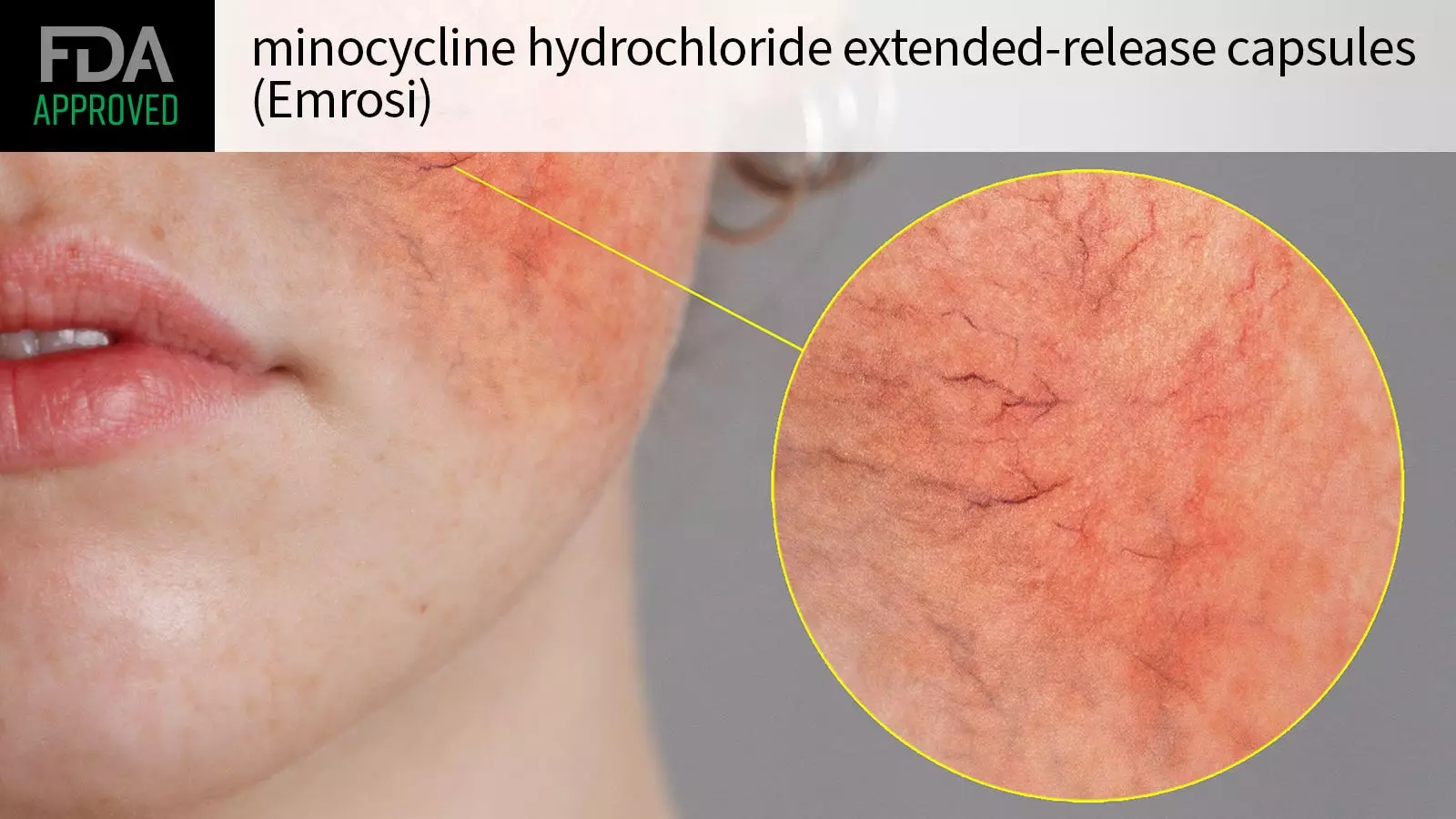
In a significant advancement for the treatment of rosacea, the U.S. Food and Drug Administration (FDA) has granted approval for minocycline hydrochloride extended-release capsules, marketed as Emrosi. This decision, announced by Journey Medical, specifically targets the inflammatory lesions associated with rosacea in adults, offering new hope to those suffering from this chronic skin condition that affects millions. The approval stems from robust clinical evidence derived from two comprehensive phase III trials that demonstrated the drug’s efficacy over existing treatments.
Clinical Trials: A Closer Look
The foundation of Emrosi’s approval lies in two multicenter trials, MVOR-1 and MVOR-2, which involved a total of 653 adult participants diagnosed with papulopustular rosacea. This common form of rosacea is characterized by the presence of inflamed papules and pustules, significantly impacting the quality of life. In these trials, participants were randomly assigned to receive daily dosages of either minocycline hydrochloride at 40 mg, the established treatment doxycycline, or a placebo over the course of 16 weeks.
At the onset of these trials, participants exhibited a minimum of 15 inflammatory lesions and scores on the Investigator’s Global Assessment (IGA) indicating moderate to severe rosacea. The results at the conclusion of the study period were compelling; a notable 65% of those treated with minocycline hydrochloride achieved an IGA score reflecting clear or near-clear skin compared to 46% and 31% for doxycycline and placebo, respectively, in the first trial. The second trial echoed similar findings, reinforcing the superiority of minocycline in reducing both the active lesions and the patient’s overall severity of disease.
The Safety Profile of Minocycline
As with any pharmaceutical agent, the safety profile of minocycline hydrochloride is important to consider. The prescribing information highlighted dyspepsia as the most frequently reported adverse effect, occurring in 2% of patients, whereas no such cases were reported in the placebo group. The data around minocycline hydrochloride also raised significant safety considerations. Notably, the medication is contraindicated for individuals with past hypersensitive reactions to tetracyclines due to serious potential side effects, including anaphylaxis, severe skin reactions, and even drug-induced systemic responses.
Furthermore, there are critical warnings related to usage in special populations, particularly pregnant women and children, as the drug can result in permanent dental staining and adversely affect bone development. The risks of Clostridioides difficile-associated diarrhea, liver toxicity, and various central nervous system effects add layers of complexity for clinicians considering this treatment.
Journey Medical anticipates that Emrosi will become available for patients in the first half of 2025, a development eagerly awaited by dermatologists and patients alike. The approval represents not just a new option in the fight against rosacea but also a reminder of the importance of robust clinical trials in establishing safe and effective treatments. As healthcare providers weigh the benefits of Emrosi against its potential risks, the drug’s launch promises to usher in a new era of management for those grappling with the discomfort and social stigma often associated with rosacea.

Leave a Reply