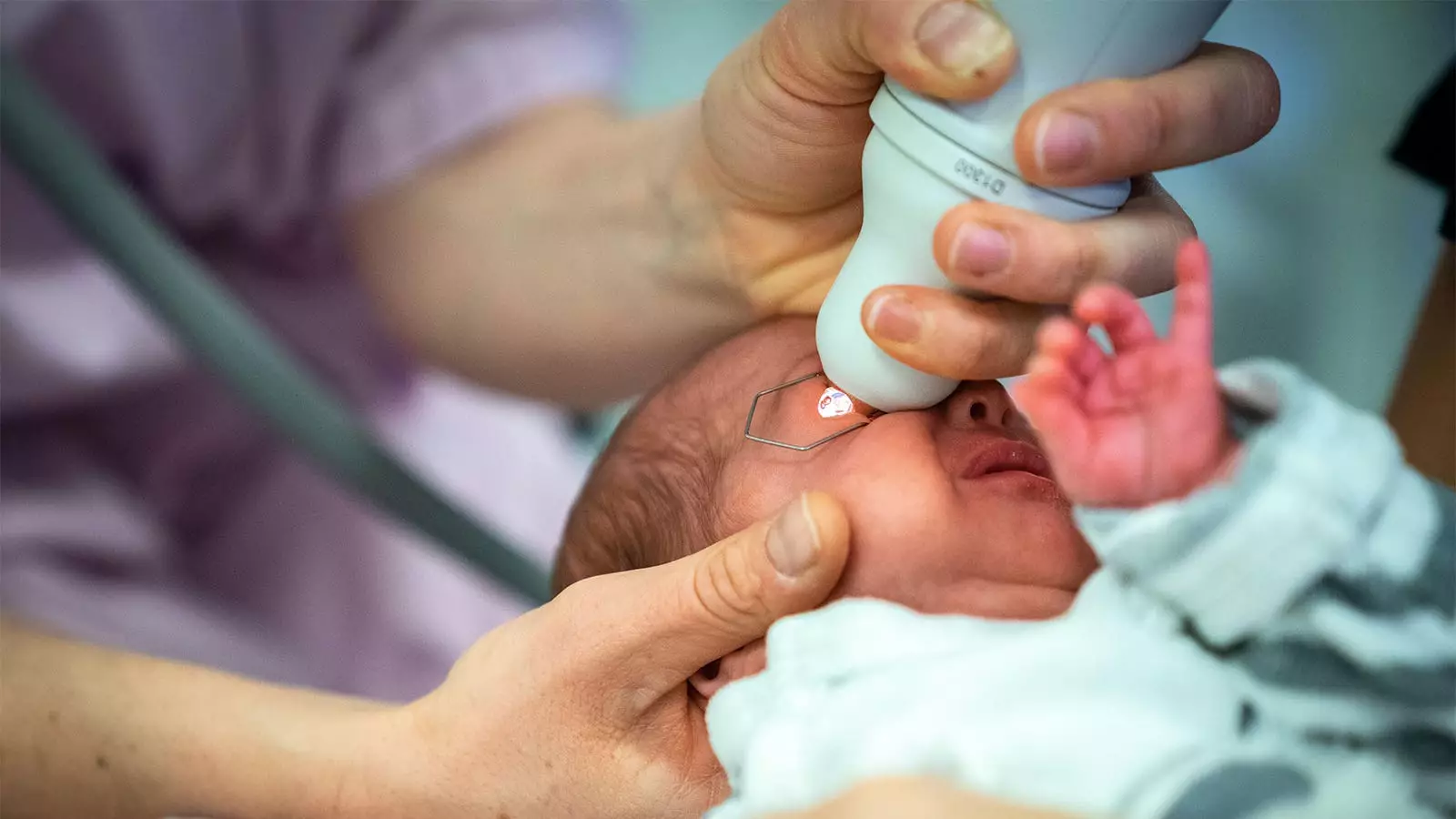
Retinopathy of prematurity (ROP) is a severe eye disorder affecting preterm infants, leading to potential vision loss and other systemic complications. Traditionally, monitoring these vulnerable patients requires the administration of mydriatic eye drops to dilate the pupils. However, concerns about adverse effects associated with standard mydriatic agents, especially in this fragile population, have prompted the exploration of alternative approaches. Recent findings suggest that mydriatic microdrops may revolutionize the current practice in ROP screening, potentially offering enhanced safety and efficacy.
The conventional mydriatic drops, while effective in pupil dilation, have raised alarm due to their association with various systemic adverse events. Preterm infants, owing to their low body mass and underdeveloped physiological systems, are particularly susceptible to these side effects. The use of standard drops has been linked to cardiorespiratory problems and gastrointestinal complications, as evidenced by numerous anecdotal reports from NICU staff monitoring these infants during and after eye examinations. These concerns underline the pressing need for safer options to facilitate necessary screenings without imposing undue risks on delicate neonates.
A recent clinical trial conducted by Asimina Mataftsi, MD, PhD, and a team from Aristotle University of Thessaloniki, provides a compelling case for the efficacy of mydriatic microdrops in ROP screening. The randomized study included 83 preterm infants and compared the effects of microdrops to traditional eye drops. Findings indicated that microdrops demonstrated superior mydriatic efficacy at the 45-minute mark, achieving a mean dilation difference that was statistically significant (mean difference 0.12, P=0.008). This suggests that microdrops may not only be a safer alternative but also a more effective option for achieving the necessary pupil dilation required for ROP screening.
In addition to their efficacy, the study highlighted the physiological impact of using mydriatic microdrops versus standard drops. Notably, lower levels of oxygen saturation were recorded in infants who received the traditional drops at 45 and 90 minutes post-administration, alongside a higher frequency of hypertensive episodes when compared to those treated with microdrops. The median percentage of hypertensive episodes, measured at 0.10% for microdrops versus 0.14% for standard drops, provides further evidence of the potential safety benefits offered by the microdrop approach. These findings resonate with earlier studies noting that ROP examinations correlate with increased apnea events and other systemic issues.
The advent of mydriatic microdrops holds transformative potential for the management of ROP in preterm infants. As innovations in neonatal care seek to enhance safety and efficacy, the incorporation of microdrops could address the longstanding challenges associated with mydriatic drug administration. The findings from Mataftsi’s study suggest that not only can microdrops achieve adequate pupil dilation, but they may also mitigate the risks of serious adverse effects traditionally associated with standard drops, thereby fostering better outcomes in this vulnerable population.
Future Research Directions
While the results are promising, Mataftsi and colleagues have acknowledged certain limitations within their study, including gaps in safety data for a significant subset of participants. This highlights the necessity for ongoing research to further validate these findings within larger, more diverse populations and under varying clinical conditions. Future investigations should aim to address the long-term consequences of using microdrops, as well as investigate their potential adaptability in different clinical settings.
The introduction of mydriatic microdrops represents a critical advancement in the approach to ROP screening. By diminishing the risk of adverse system-wide effects while maintaining efficacy in pupil dilation, this method could pave the way for safer neonatal eye care practices. As researchers continue to unveil the benefits and broaden the application of mydriatic microdrops, the healthcare community may soon witness a paradigm shift in the management of retinopathy of prematurity, ultimately safeguarding the health and vision of preterm infants.

Leave a Reply