Ovarian cancer remains one of the deadliest malignancies for women, particularly the aggressive high-grade serous ovarian carcinoma (HGSOC), which often goes undetected until it reaches an advanced stage. A recent study focusing on the cellular origins of this cancer in mice provides critical insights that could revolutionize early detection and treatment methods for humans. By pinpointing the cells involved in the initial formation of HGSOC, researchers may pave the way for innovative diagnostics and targeted therapies that could ultimately save lives.
One of the significant obstacles in combating HGSOC is the lack of identifiable symptoms in its early stages. Most patients diagnosed with this cancer face a grim prognosis since around 80% of cases are discovered at an advanced level, where treatment options become limited and less effective. By the time of diagnosis, many patients are already in grave danger, highlighting the urgent need for a better understanding of how this cancer develops and progresses.
Historically, the fallopian tubes have been overlooked as a potential site of origin for many ovarian cancers, with the assumption that these cancers began directly within the ovaries. However, research conducted over the past decade has shifted this paradigm, indicating that HGSOC may primarily originate in the fallopian tubes. This insight underscores the importance of exploring the oviducts (the tubes widening from the uterus to the ovaries in females) as a critical area for early detection.
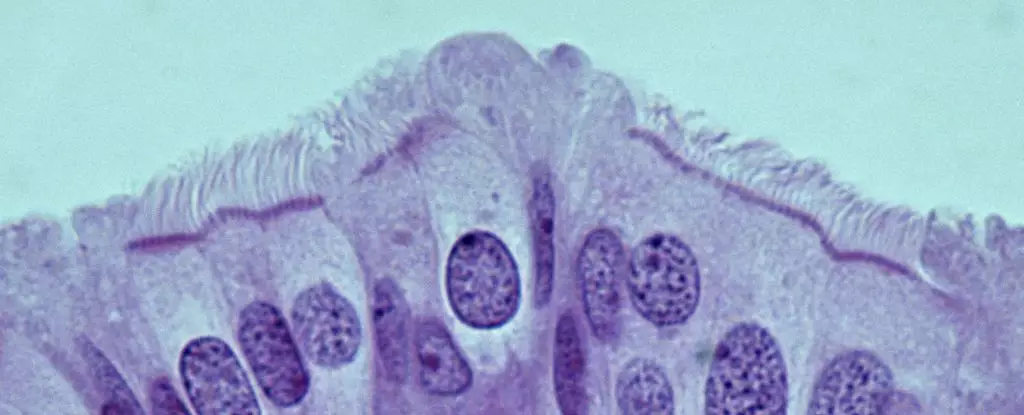
A team of researchers, led by Alexander Nikitin from Cornell University, has made significant strides in identifying the specific cell types within the oviduct that are linked to the development of HGSOC. Their groundbreaking study has cataloged the various cell populations present in the oviduct for the first time. This effort could radically advance our understanding of which cells are culpable in the malignant transformation associated with ovarian cancer.
The researchers found that pre-ciliated cells—rather than stem cells—are responsible for cancer susceptibility in the oviducts of mice. These transitional cells undergo changes to form ciliated cells, which are vital for transporting oocytes through the reproductive tract. The discovery highlights a specific vulnerability in the process of ciliogenesis, whereby two genetic mutations tied to HGSOC cause dysfunction within these pre-ciliated cells. This malfunction appears critical in facilitating the onset of cancer, thereby establishing a direct link between the developmental mechanisms in the oviduct and the emergence of ovarian cancer.
The implications of this research are profound. Should the findings in mice hold true for human physiology, identifying the cancer-prone pre-ciliated cells could revolutionize how clinicians approach ovarian cancer screening and prevention. The enhanced ability to detect high-grade serous ovarian carcinomas at earlier, more treatable stages could significantly improve patient outcomes and survival rates.
Furthermore, understanding how the regulation of ciliary formation relates to ovarian cancer opens new avenues for research. If researchers can further clarify the genetic underpinnings associated with HGSOC and how they interplay with pre-ciliated cells, it may become possible to identify novel diagnostic markers as well as therapeutic targets.
The recent identification of specific cell types in the oviduct associated with HGSOC is a pivotal step forward in the quest to improve early detection and treatment outcomes for ovarian cancer. Continued research into the cellular and genetic mechanisms at play can help ensure that critical insights lead to actionable changes in medical practice, ultimately aiding in the fight against this deadly disease.

Leave a Reply