Every moment, our bodies engage in an incessant cycle of life and death at a microscopic level. With approximately a million cells perishing every second, one may wonder—what happens to these cells after they die? Recent research illuminated a remarkable phenomenon in which living stem cells voraciously consume their deceased counterparts, showcasing a unique self-cleaning mechanism within the body. This fascinating process not only purges waste but also ensures the survival and functionality of the remaining cells, emphasizing a profound interdependency within our cellular architecture.
Through extensive study, scientists have observed that certain stem cells in mammals demonstrate a somewhat ‘cannibalistic’ behavior, promoting a cleanup method that benefits their immediate environment. Researchers from The Rockefeller University discovered that living stem cells possess sensitive receptors specifically tuned to detect the biochemical signals from dying cells. These receptors act like a biological alarm system, alerting neighboring healthy cells of nearby death through subtle ‘smells’ emitted by their deceased neighbors. According to cellular biologist Katherine Stewart, “The mechanism only functions when each receptor picks up the signal it is attuned to.” This unique detection system underscores the delicate balance maintaining the health of cellular communities.
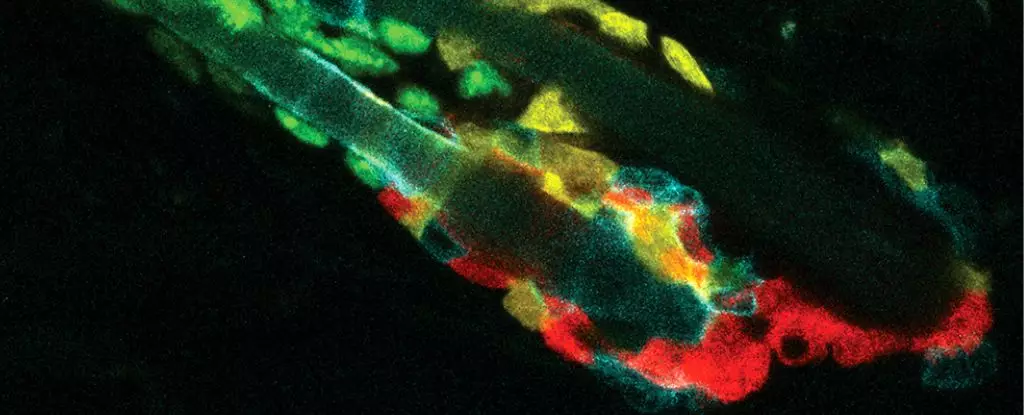
This phenomenon was particularly evident in studies examining the hair follicles of aging mice. Once hair follicle stem cells (HFSCs) succumb to death, neighboring HFSCs promptly move in to consume these fallen comrades, significantly outpacing the response of immune cells such as macrophages. Thus, rather than waiting for external cleanup agents, these stem cells exhibit rapid, efficient self-governance, effectively shielding themselves from potential inflammatory reactions triggered by external immune responses.
The implications of this discovery are profound. The rapid clearance of dead HFSCs seems to serve a critical purpose; by consuming their lifeless neighbors, these living stem cells can stave off inflammation that would otherwise disrupt the delicate functions of hair growth and tissue maintenance. Stewart highlights that, although macrophages are plentiful within the skin of mice, nearby HFSCs still prioritize their own when it comes to cleanup duties. “I was very surprised to find that the hair follicle stem cells were actually the first responders,” Stevens revealed of the findings that prioritized cellular collaboration over traditional immunological response.
When HFSCs fail to consume their dead neighbors, the residual fragments may interfere with vital aspects of stem cell functionality. The ability for HFSCs to collectively consume their dead signifies a sustainable recycling method, which serves as an energy source while maintaining the integrity of the entire stem cell pool. On the contrary, a failure to support one another by consuming dead cells could lead to long-term dysfunction, showcasing the importance of this cellular collaboration in maintaining bodily health.
What drives this intricate process? Researchers uncovered a sophisticated regulatory mechanism involving two distinct receptors present on the surface of HFSCs. One receptor is attuned to a “find me” lipid signal from dying cells, while the other responds to a growth-promoting retinoic acid from healthier cells. This finely-tuned system operates much like a biological toggle switch—stimulated by death and nurtured by life. Upon detection of dead cells, stem cells react accordingly, rapidly consuming the debris and transitioning back to their primary functions as soon as their task is completed.
Stewart elegantly explains this dual signaling: “When a dying cell triggers the mechanism to begin, and when there are no dead cells left, the lipid signal disappears, leaving only the retinoic acid signal from the healthy cells… It’s so elegant in its simplicity.” The brevity and precision of this signaling pathway highlight the evolutionary brilliance of cellular dynamics.
While this research mainly focuses on hair follicle stem cells in mice, experts believe that similar processes could be at play in other tissues across mammalian organisms. The potential for this ‘self-eating’ mechanism to apply to various biological systems prompts a wealth of questions begging further exploration. As researchers embark on new studies to test these theories, the implications of understanding this efficient cellular recycling system could lead to groundbreaking advancements in regenerative medicine and tissue engineering.
Through the lens of cell death and consumption, we witness not only a unique survival tactic but also an example of the inherent cooperation that governs life at its most fundamental level. In our pursuit to decipher the complexities of biology, this new understanding of stem cell behavior may open corridors to insight and innovation for future studies in medicine and longevity.

Leave a Reply